Work :
`=>` First of all, let us concentrate on the nature of work a system can do. We will consider only mechanical work i.e., pressure-volume work.
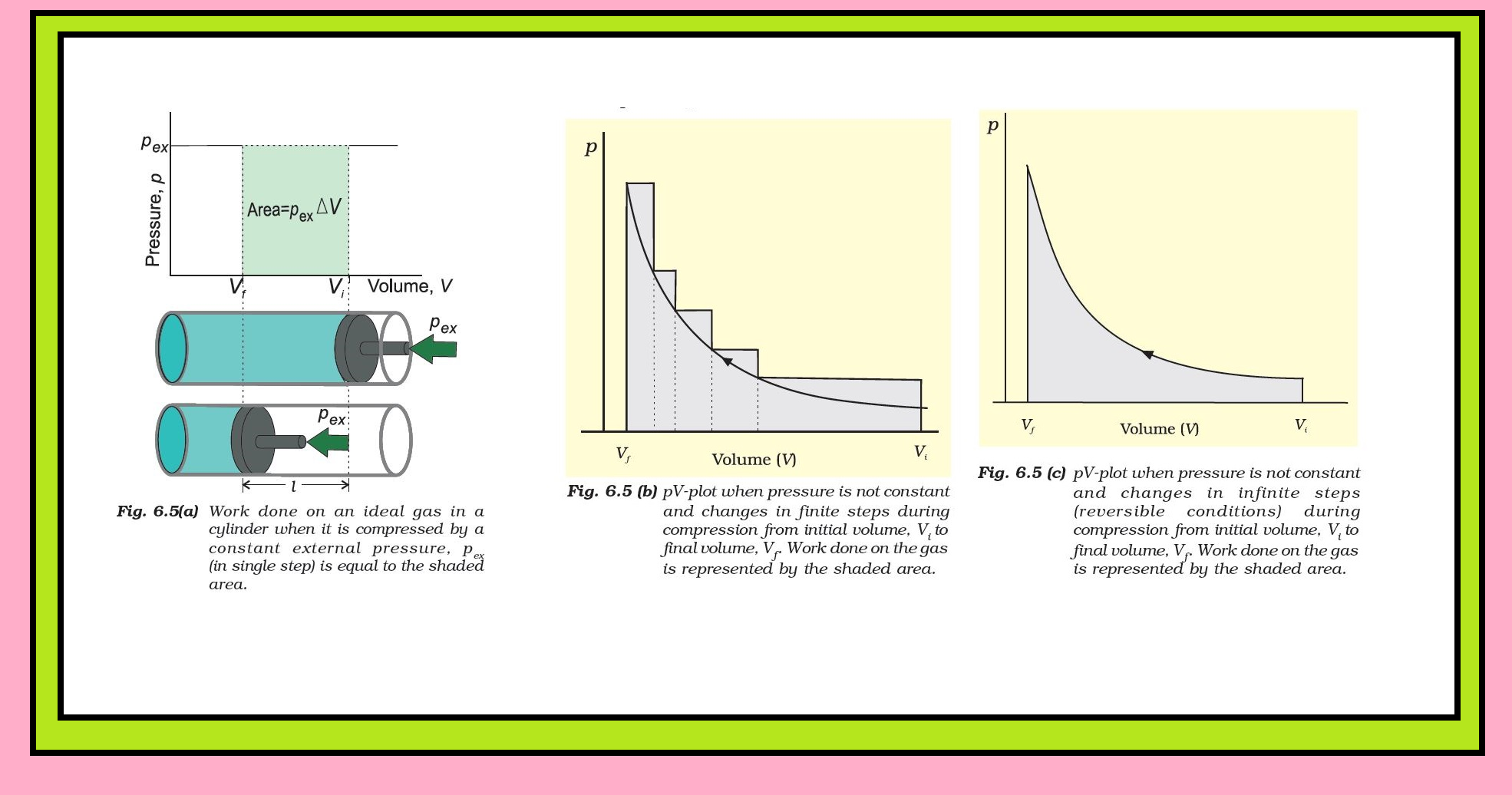
`=>` For understanding pressure-volume work, let us consider a cylinder which contains one mole of an ideal gas fitted with a frictionless piston.
● Total volume of the gas is `color{red}(V_i)` and pressure of the gas inside is `p`.
● If external pressure is `color{red}(p_(ex))` which is greater than `p`, piston is moved inward till the pressure inside becomes equal to `color{red}(p_(ex))`.
● Let this change be achieved in a single step and the final volume be `color{red}(V_f)`.
● During this compression, suppose piston moves a distance, `color{red}(l)` and cross-sectional area of the piston is `A` [Fig. 6.5(a)].
●Then, volume change `color{red}(= l × A = ΔV = (V_f – V_i ))`
We also know, pressure `color{red}(= text(force)/text(area))`
Therefore, force on the piston ` color{red}(= p_(ex) . A)`
● If `color{red}(w)` is the work done on the system by movement of the piston then `w= text(force) xx text(distance) = p_(ex) . A l`
`color{red}(= p_(ex) . (- DeltaV) = - p_(ex) DeltaV = - p_(ex) (V_f - V_i))` .......(6.2)
● The negative sign of this expression is required to obtain conventional sign for `w`, which will be positive.
● It indicates that in case of compression work is done on the system. Here `color{red}((V_f – V_i ))` will be negative and negative multiplied by negative will be positive. Hence the sign obtained for the work will be positive.
`=>` If the pressure is not constant at every stage of compression, but changes in number of finite steps, work done on the gas will be
summed over all the steps and will be equal to `color{red}(−Σ pΔV)` [Fig. 6.5 (b)]
`=>` If the pressure is not constant but changes during the process such that it is always infinitesimally greater than the pressure of the gas, then, at each stage of compression, the volume decreases by an infinitesimal amount, `dV`. In such a case we can calculate the work done on the gas by the relation
`color{red}(w = - int_(V_i)^(V_f) p_(ex) dV)` .....................(6.3)
Here, `color{red}(p_(ex))` at each stage is equal to `color{red}((p_(i n) + dp))` in case of compression [Fig. 6.5(c)].
`=>` In an expansion process under similar conditions, the external pressure is always less than the pressure of the system i.e., `color{red}(p_(ex) = (p_(i n)– dp))`. In general case we can write, `color{red}(p_(ex) = (p_(i n) pm dp))`. Such processes are called `color{red}("reversible processes")`.
`color{green}("Reversible Process ")` A process or change is said to be reversible, if a change is brought out in such a way that the process could, at any moment, be reversed by an infinitesimal change. A reversible process proceeds infinitely slowly by a series of equilibrium states such that system and the surroundings are always in near equilibrium with each other.
● Processes other than reversible processes are known as `color{red}("irreversible processes")`.
`=>` In chemistry, we face problems that can be solved if we relate the work term to the internal pressure of the system. We can relate work to internal pressure of the system under reversible conditions by writing equation 6.3 as follows :
`color{red}(w_(rev) = - int_(V_i)^(V_f) p_(ex) dV)`
Since `color{red}(dp × dV)` is very small we can write
`color{red}(w_(rev) = - int_(V_i)^(V_f) p_(i n)dV)` ..........(6.4)
● Now, the pressure of the gas (`color{red}(p_(i n))` which we can write as `color{red}(p)` now) can be expressed in terms of its volume through gas equation.
For `n` mol of an ideal gas i.e., `color{red}(pV =nRT)`
`=> color{red}(p = (n RT)/V)`
● Therefore, at constant temperature (isothermal process),
`color{red}(w_(rev) = - int_(V_i)^(V_f) n RT/V) dV`
`=color{red}( – 2.303 nRT log \ \ V_f/V_i)` ............(6.5)
`color{green}("Free expansion ")` Expansion of a gas in vacuum `color{red}((p_(ex) = 0))` is called free expansion. No work is done during free expansion of an ideal gas whether the process is reversible or irreversible (equation 6.2 and 6.3).
`=>` Now, we can write equation 6.1 in number of ways depending on the type of processes.
● Let us substitute `color{red}(w = – p_(ex)ΔV)` (eq. 6.2) in equation 6.1, and we get
`color{red}(ΔU = q − p_(ex) ΔV)`
● If a process is carried out at constant volume `(ΔV = 0),` then
`color{red}(ΔU = q_V)`
the subscript `color{red}(V)` in `color{red}(q_V)` denotes that heat is supplied at constant volume.
`=>` For understanding pressure-volume work, let us consider a cylinder which contains one mole of an ideal gas fitted with a frictionless piston.
● Total volume of the gas is `color{red}(V_i)` and pressure of the gas inside is `p`.
● If external pressure is `color{red}(p_(ex))` which is greater than `p`, piston is moved inward till the pressure inside becomes equal to `color{red}(p_(ex))`.
● Let this change be achieved in a single step and the final volume be `color{red}(V_f)`.
● During this compression, suppose piston moves a distance, `color{red}(l)` and cross-sectional area of the piston is `A` [Fig. 6.5(a)].
●Then, volume change `color{red}(= l × A = ΔV = (V_f – V_i ))`
We also know, pressure `color{red}(= text(force)/text(area))`
Therefore, force on the piston ` color{red}(= p_(ex) . A)`
● If `color{red}(w)` is the work done on the system by movement of the piston then `w= text(force) xx text(distance) = p_(ex) . A l`
`color{red}(= p_(ex) . (- DeltaV) = - p_(ex) DeltaV = - p_(ex) (V_f - V_i))` .......(6.2)
● The negative sign of this expression is required to obtain conventional sign for `w`, which will be positive.
● It indicates that in case of compression work is done on the system. Here `color{red}((V_f – V_i ))` will be negative and negative multiplied by negative will be positive. Hence the sign obtained for the work will be positive.
`=>` If the pressure is not constant at every stage of compression, but changes in number of finite steps, work done on the gas will be
summed over all the steps and will be equal to `color{red}(−Σ pΔV)` [Fig. 6.5 (b)]
`=>` If the pressure is not constant but changes during the process such that it is always infinitesimally greater than the pressure of the gas, then, at each stage of compression, the volume decreases by an infinitesimal amount, `dV`. In such a case we can calculate the work done on the gas by the relation
`color{red}(w = - int_(V_i)^(V_f) p_(ex) dV)` .....................(6.3)
Here, `color{red}(p_(ex))` at each stage is equal to `color{red}((p_(i n) + dp))` in case of compression [Fig. 6.5(c)].
`=>` In an expansion process under similar conditions, the external pressure is always less than the pressure of the system i.e., `color{red}(p_(ex) = (p_(i n)– dp))`. In general case we can write, `color{red}(p_(ex) = (p_(i n) pm dp))`. Such processes are called `color{red}("reversible processes")`.
`color{green}("Reversible Process ")` A process or change is said to be reversible, if a change is brought out in such a way that the process could, at any moment, be reversed by an infinitesimal change. A reversible process proceeds infinitely slowly by a series of equilibrium states such that system and the surroundings are always in near equilibrium with each other.
● Processes other than reversible processes are known as `color{red}("irreversible processes")`.
`=>` In chemistry, we face problems that can be solved if we relate the work term to the internal pressure of the system. We can relate work to internal pressure of the system under reversible conditions by writing equation 6.3 as follows :
`color{red}(w_(rev) = - int_(V_i)^(V_f) p_(ex) dV)`
Since `color{red}(dp × dV)` is very small we can write
`color{red}(w_(rev) = - int_(V_i)^(V_f) p_(i n)dV)` ..........(6.4)
● Now, the pressure of the gas (`color{red}(p_(i n))` which we can write as `color{red}(p)` now) can be expressed in terms of its volume through gas equation.
For `n` mol of an ideal gas i.e., `color{red}(pV =nRT)`
`=> color{red}(p = (n RT)/V)`
● Therefore, at constant temperature (isothermal process),
`color{red}(w_(rev) = - int_(V_i)^(V_f) n RT/V) dV`
`=color{red}( – 2.303 nRT log \ \ V_f/V_i)` ............(6.5)
`color{green}("Free expansion ")` Expansion of a gas in vacuum `color{red}((p_(ex) = 0))` is called free expansion. No work is done during free expansion of an ideal gas whether the process is reversible or irreversible (equation 6.2 and 6.3).
`=>` Now, we can write equation 6.1 in number of ways depending on the type of processes.
● Let us substitute `color{red}(w = – p_(ex)ΔV)` (eq. 6.2) in equation 6.1, and we get
`color{red}(ΔU = q − p_(ex) ΔV)`
● If a process is carried out at constant volume `(ΔV = 0),` then
`color{red}(ΔU = q_V)`
the subscript `color{red}(V)` in `color{red}(q_V)` denotes that heat is supplied at constant volume.





